Technologies
BioWa serves as the exclusive licensor of POTELLIGENT® and COMPLEGENT® Technologies to pharmaceutical companies and biotech companies all over the world.
POTELLIGENT®
The elimination of fucose from sugar chains on an antibody has forever changed the art and science of therapeutics. BioWa has harnessed the ability to create 100% fucose-free monoclonal antibodies, thereby enhancing Antibody Dependent Cellular Cytotoxicity (ADCC), the critical factor in anti-tumor activity. We call it a powerful ripple effect.
This revolutionary POTELLIGENT® Technology promises to deliver the ultimate antibody warriors in tumor annihilation. POTELLIGENT® Technology is simple to use and completely compatible with your manufacturing process. Results have shown dramatic enhancement of ADCC activity and increased binding affinity to the Fc Receptor to overcome genetic polymorphism, a major obstacle to the effectiveness of current monoclonal therapies.
BioWa is the exclusive provider of POTELLIGENT® Technology worldwide.
Breakthrough antibody technology
- Dramatically enhances the potency and efficacy of antibodies. Clinical results with POTELLIGENT®-enhanced antibodies are expected to show higher efficacy in human patients when compared with antibodies that have not been enhanced.
- Increases Fc receptor binding – overcomes the problem of low clinical responses due to genetic differences in the Fc receptor.
- Lowers the effective therapeutic does of your antibodies – the potential to deliver much more with much less.
- POTELLIGENT® Technology creates antibodies that are expected to be proven safe and well tolerated with no immunogenic concerns.
- Requires no change in your current manufacturing process – BioWa's proprietary technology works seamlessly with the systems and processes you already have in place.
- Reduces costs of goods (COGs).
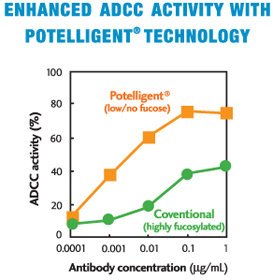
The scientists at Kyowa Hakko Kogyo Co., Ltd. (now, Kyowa Kirin Co., Ltd.) discovered that when an antibody had a reduced amount of fucose in its sugar chains, it exhibited much higher Antibody Dependent Cellular Cytotoxicity (ADCC) activity as compared to a highly-fucosylated conventional antibody.
Soon after, it was demonstrated that the mechanism behind the enhanced ADCC of a low/no-fucose antibody was its increased affinity to FcγRIIIa (CD16), the major Fc receptor for ADCC in humans.
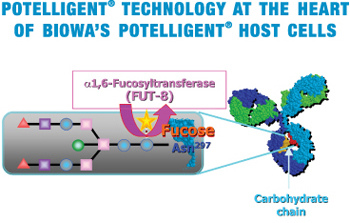
To take advantage of the above finding, our scientists strategically knocked out a gene called FUT8 that is responsible for the fucose addition to sugar chains in order to create a new production method for fucose-free antibodies in CHO cells. Unprecedented, through tenacious effort, our scientist finally created the FUT8-knock-out CHO cells (POTELLIGENT® Cells).
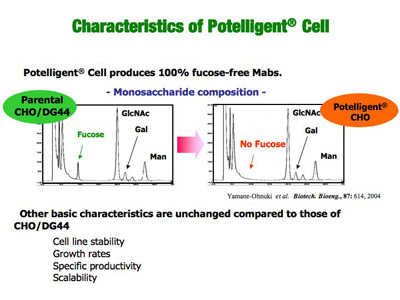
POTELLIGENT® Cells are shown to produce fucose-free, thereby ADCC-enhanced antibodies (POTELLIGENT® Mabs), with unchanged basic properties (stability, growth rate, productivity and scalability) compared to parental CHO cells.
POTELLIGENT® Cells have cleared bio-safety testing, are being reposited under GMP, and are ready to use for our partners.
To date, a number of benefits of POTELLIGENT® Mabs have been elucidated
- Show enhanced ADCC activity even with the low-ADCC allotype of CD16, overcoming CD16 polymorphism issue
- Exert potent cytotoxicity against low-density antigens
- Efficiently work in whole blood
- Exhibit higher efficacy even at a lower dose than a conventional Mab dose (Fig. 3)
- Retain desirable features of Mabs, such as Protein A biding, protein stability, and PK profile
Several POTELLIGENT® Mabs are currently in ongoing clinical trials. We expect clinical trial results to show immense benefits in efficacy and potency. We also anticipate these antibodies to be safe and well tolerated in humans.
References:
- Beck A et al., Mabs, 4, 419-25, 2012
- Subramaniam JM et al., Drugs, 72, 1293-8, 2012
- Tobinai K et al., Curr Hematol Malig Rep, 7, 235-40, 2012
- Ishida T et al., J Clin Oncol, 30, 837-42, 2012
- Ward E et al., Br J Haematol, 155, 426-37, 2011
- Ishida T et al., Int J Hematol, 94, 443-52 2011
- Matsushita T, Korean J Hematol, 46, 148-50, 2011
- Antoniu SA, Curr Opin Mol Ther, 12, 770-9, 2010
- Tobinai K, Semin Hematol, 47 Suppl 1:S5-7, 2010
- Kubota T et al., Anticancer Res, 30 3397-405, 2010
- Herbst R et al., J Pharmacol Exp Ther, 335, 213-22, 2010
- Junttila TT et al., Cancer Res, 70, 4481-9, 2010
- Busse WW et al., J Allergy Clin Immunol, 125, 1237-44, 2010
- Kolbeck R et al., J Allergy Clin Immunol, 125, 1344-53, 2010
- Nakagawa T et al., Leuk Res, 34, 666-671, 2010
- Yamamoto K et al., J Clin Oncol, 28, 1591-8, 2010
- Ishii T et al., Clin Cancer Res, 16, 1520-31, 2010
- Cardarelli PM et al., Cancer Immunol Immunother, 59, 257-65, 2010
- Ito A et al., J Immunol. 2009, 183, 4782-91, 2009
- Ito A et al., Cancer Immunol Immunother, 58, 1195-206, 2009
- Yamane-Ohnuki N, MAbs, 1, 230-6, 2009
- Cardarelli PM et al., Clin Cancer Res, 15, 3376-83, 2009
- Natsume A et al., Drug Des Devel Ther, 3, 7-16, 2009
- Shibata-Koyama M et al., Exp Hematol, 37, 309-21, 2009
- Shibata-Koyama M et al., Glycobiology, 19, 126-34, 2009
- Kubota T et al., Cancer Sci, 100, 1566-72, 2009
- Yamane-Ohnuki N et al., Methods Mol Biol, 435, 1-16, 2008
- Yano H et al., Br J Haematol, 140, 586-9, 2008
- Mori K et al., Cytotechnology, 55, 109-14, 2007
- Yano H et al., Clin Cancer Res, 13, 6494-500, 2007
- Masuda K et al., Mol Immunol, 44, 3122-31, 2007
- Matsumiya S et al., J Mol Biol, 368, 767-79, 2007
- Kanda Y et al., Glycobiology, 17, 104-18, 2006
- Shoji-Hosaka E et al., J Biochem, 140, 777-83, 2006
- Natsume A et al., J Biochem, 140, 359-68, 2006
- Satoh M et al., Expert Opin Biol Ther, 6, 1161-73, 2006
- Kanda Y et al., Biotech Bioeng, 94, 680-8, 2006
- Iida S et al., Clin Cancer Res, 12, 2879-87, 2006
- Niwa R et al., J Immunol Methods, 306, 151-60, 2005
- Natsume A et al., J Immunol Methods, 306, 93-103, 2005
- Niwa R et al., Clin Cancer Res, 11, 2327-36, 2005
- Mori K et al., Biotech Bioeng, 88, 901-8, 2004
- Niwa R et al., Clin Cancer Res, 10, 6248-55, 2004
- Yamane-Ohnuki N, Biotech Bioeng, 87, 614-22, 2004
- Okazaki A et al., J Mol Biol, 336, 1239-49, 2004
- Niwa R et al., Cancer Res, 64, 2127-33, 2004
- Shinkawa T et al., J Bio Chem, 278, 3466-73, 2003
POTELLIGENT® Website
COMPLEGENT®
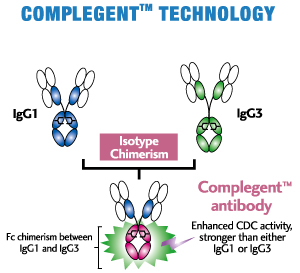
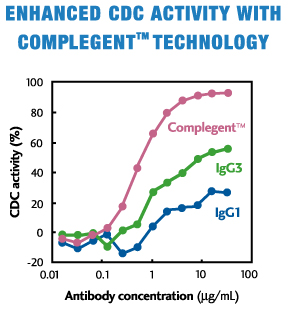
CCOMPLEGENT® Technology is a new technology developed by Kyowa Hakko Kogyo Co., Ltd. (now, Kyowa Kirin Co., Ltd.) that enhances one of the major mechanisms of action of an antibody, CDC. With an approach called isotype chimerism, in which portions of IgG3, an antibody's isotype, are introduced into corresponding regions of IgG1, the standard isotype for therapeutic antibodies, COMPLEGENT® Technology significantly enhances CDC activity beyond that of either IgG1 or IgG3, while retaining the desirable features of IgG1, such as ADCC, PK profile and Protein A binding. In addition, it can be used together with POTELLIGENT® Technology, creating an even superior therapeutic Mab (AccretaMab®) with enhanced ADCC and CDC activities.
The Benefits of COMPLEGENT® Technology
- Enhances CDC activity more than 10-fold by Fc chimerism between IgG1 and IgG3
- Retains desirable features of IgG1, including;
- ADCC activity
- PK profile
- Protein A binding
- Taps the naturally occurring sequence from IgG1 and IgG3
References:
- Kaneko E et al., Biodrugs, 25, 1-11, 2011
- Sato F et al., Cancer Immunol Immunother., 59, 1791-800, 2010
- Natsume A et al., Drug Des Devel Ther, 3, 7-16, 2009
- Natsume A et al., Cancer Sci., 100, 2411-8, 2009
- Kubota T et al., Cancer Sci, 100, 1566-72, 2009
- Natsume A et al., Cancer Res., 68, 3863-72, 2008
AccretaMab®
AccretaMab® - Antibodies Created Using Both POTELLIGENT® and COMPLEGENT® Technologies - the ultimate enhanced antibody therapeutics (with both enhanced ADCC and CDC activities)